Clinical Trial Process Automation
Accelerate study start-up, reduce errors, and streamline regulatory compliance — all without paper, spreadsheets, or custom coding. Nutrient delivers modern tools to transform every phase of clinical trials for speed, accuracy, and oversight.
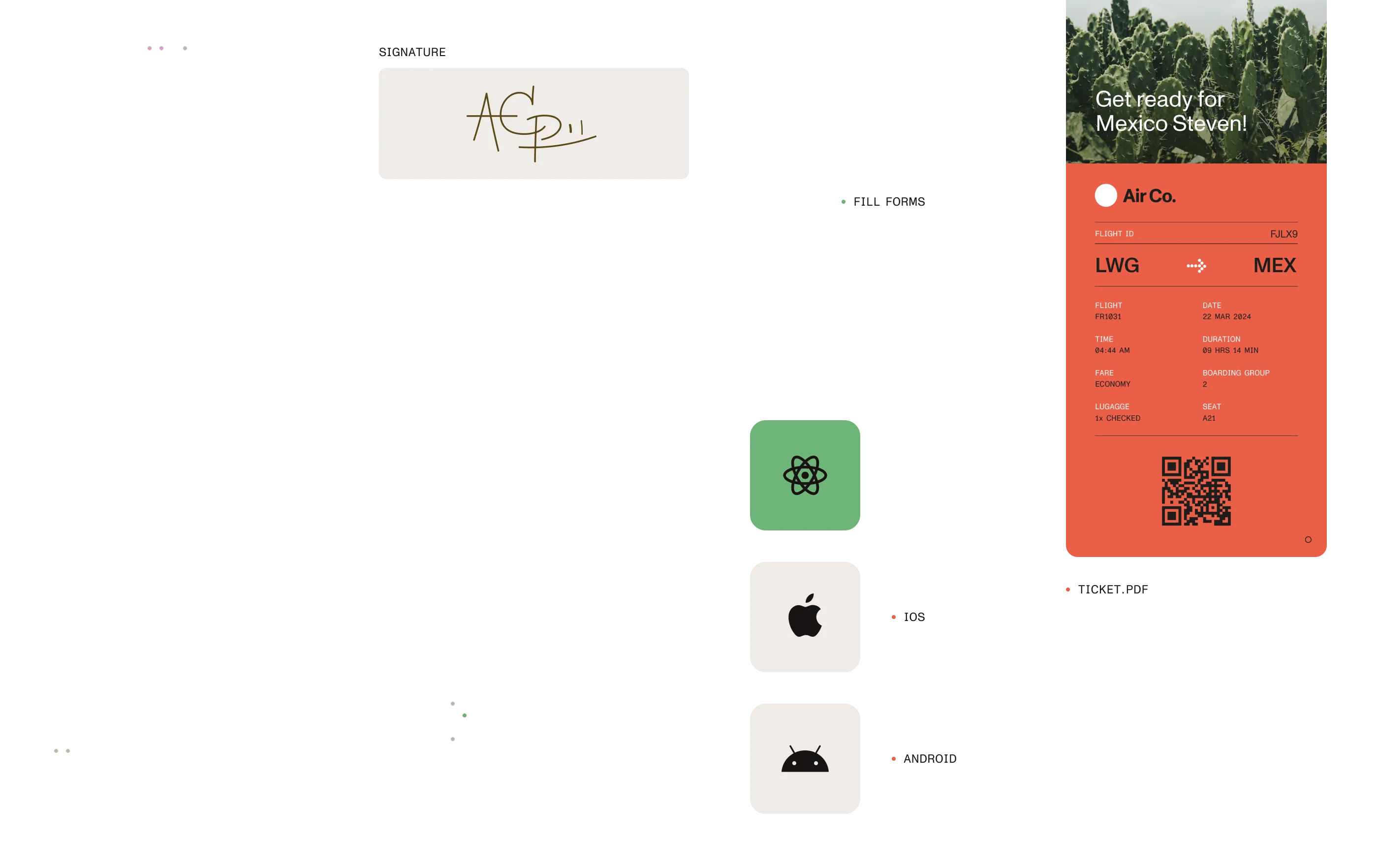

How We help
How we help
Digitize manual trial workflows
Stop managing clinical studies with disparate tools. Nutrient centralizes trial processes into a unified, digital platform purpose-built for regulated research.
Align study teams and sites
Create a single source of truth for study data, approvals, and documentation — so no information is lost, and everyone’s on the same page.
Automate protocol and submission routing
Route documents and protocols for review, escalation, or approval based on role, region, or study phase, ensuring compliance every step.
Increase visibility and compliance
Monitor milestones, document status, and team progress with real-time dashboards and audit trails built for regulatory transparency.
Reduce risk of errors and delays
Automated data validation and notification workflows proactively identify issues, minimizing delays and improving study accuracy.
Scale across multiple trials and sites
Adapt Nutrient to any therapeutic area or trial phase, configuring processes once for cross-study consistency, risk controls, and traceability.
Key features
Key features
Drag-and-drop workflow builder

Design and automate trial milestones, document reviews, and principal investigator approvals — all in an intuitive visual designer tailored for clinical research.
eSource and eCRF integration

Generate responsive electronic Case Report Forms, automate data capture, and ensure high-quality data moves seamlessly into your study database.
Self-service site portal

Enable sites and investigators to submit documents, receive assignments, collaborate, and track progress — with role-based access and real-time notifications.
Audit trail & regulatory reporting

Follow every action with immutable, regulator-ready audit logs. Create dashboards, compliance reports, and export study activity for inspections.
Open process integration API

Connect with EDC, CTMS, and regulatory submission platforms using Nutrient’s open API and cloud integrations. Automate data movement and trigger protocol-driven workflows.
Role-based permissions and controls

Set granular access, automate protocol-based permissions, and keep every data interaction controlled and compliant across the trial’s lifecycle.
Explore all our low-code document solutions
Every team, workflow, and use case is different. Nutrient offers a proven suite of tools and integrations — built to work together and designed to help you get started fast. Pick the solution that best fits your document automation needs.
Document Converter
Convert files across formats (e.g., Excel to Word or PDF) in workflows that are fast, flexible, and fully automated.
Learn MoreDocument⠀ Editor
Enable inline editing of generated Word documents—right inside your browser, with no Word installation needed.
Learn MoreDocument Searchability
Make your generated or uploaded documents text-searchable with OCR processing and metadata enhancement.
Learn MoreDocument Automation
Deploy and manage scalable, secure document automation workflows behind your firewall or in your private cloud.
Learn MoreWhy Nutrient?

No-code simplicity
Empower operations teams to own automation.

Secure by design
Built for regulated industries and compliance.

Deep Microsoft 365 integration
Seamless workflows inside the tools you already use.

Fast time to value
Stand up solutions in days, not months.
Trusted by leading organizations









Benefits
Benefits
Collect consistent data, validate submissions, and route every protocol through defined, compliant workflows.
Collect consistent data, validate submissions, and route every protocol through defined, compliant workflows.
Move protocols and submissions forward with automatic routing, escalations, and reminders, cutting out manual delays.
Sync with CTMS, EDC, or eTMF platforms to keep processes unified and avoid rekeying critical data.
Embed regulatory controls and automate compliance checks, so every trial stays on track and audit ready.
Give sites and study teams one intuitive interface for submissions, approvals, and tracking progress — no IT help required.
Get started today
See how modern clinical automation can accelerate your study timelines and give your teams more time for what matters — better research.

Connect to your tools, your way
Workflow Automation integrates with your tech stack — including finance systems, procurement platforms, and approval tools — using APIs, webhooks, or SFTP. No extra middleware required.


















Frequently asked questions
Why automate clinical trial workflows?
Automation shortens timelines, minimizes human error, ensures protocol adherence, and frees up research staff to focus on patients and data quality instead of paperwork.
Do clinical operations teams need IT support to manage this?
Not usually. Most workflows can be built and managed by study coordinators or ops leads using Nutrient’s drag-and-drop tools. IT may help with deeper integrations.
Can this integrate with EDC, CTMS, or eTMF systems?
Yes. Nutrient integrates with common clinical systems so workflows can trigger or respond to actions in your EDC, CTMS, or document management platforms.
How fast can we deploy trial automations?
You can go live with targeted workflows (like consent or site setup) in days. Full trial process automation across multiple sites may take a few weeks, depending on complexity.
What’s the impact on trial timelines and data quality?
Expect fewer delays, cleaner data, better communication between teams, and faster reporting — all contributing to more successful, on-time trials.
How fast can we deploy trial automations?
You can go live with targeted workflows (like consent or site setup) in days. Full trial process automation across multiple sites may take a few weeks, depending on complexity.
Get started today with a free trial
See how modern clinical automation can accelerate your study timelines and give your teams more time for what matters — better research.
.png)




